Doping Reaction of some Nanotubes with Aluminium Atom: A Thermodynamic PM6 and ONIOM Investigation
Nasrin Zeighami1,2, Mohammad Reza Gholami3 and Asadollah Boshra4
1Department of Chemistry,Payame Noor University Maraghe,Iran.
2Young Researchers and Elite Club, Sience and Research Branch , Islamic Azad University Tehran ,Iran.
3Department of Chemistry, Sharif University of Technology Tehran, Iran.
4Nanoscience Computation Lab, Islamic Azad University, Boroujerd Branch,Lorestan.
Corresponding Author E-mail: n.zeighami@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/330139
The doping reaction of boron nitride and carbon nanotubes with aluminium atom was theoretically investigated. ONIOM method and PM6 method have been used to evaluate the thermochemistry of doping reactions of single walled boron nitride nanotubes and carbon nanotubes. The enthalpy changes, Gibbs free energy changes, of studied doping reactions were evaluated at different temperatures. All nanotubes were single-walled and finite length with hydrogen saturation in the terminal atoms. The thermodynamic calculations based on the ONIOM and PM6 levels results showed (8,0)CNT is the best candidate for Al-doping reaction.result suggest the aluminum doped boron nitride nanotubes and carbon nanotubes may be considered the proper carries for the drug delivery.
KEYWORDS:ONIOM methods; Semiempirical method; Nanotubes; Doped nanotubes; Thermodynamic functions
Download this article as:| Copy the following to cite this article: Zeighami N, Gholami M. R, Boshra A. Doping Reaction of some Nanotubes with Aluminium Atom: A Thermodynamic PM6 and ONIOM Investigation. Orient J Chem 2017;33(1). |
| Copy the following to cite this URL: Zeighami N, Gholami M. R, Boshra A. Doping Reaction of some Nanotubes with Aluminium Atom: A Thermodynamic PM6 and ONIOM Investigation. Orient J Chem 2017;33(1). Available from: http://www.orientjchem.org/?p=30491 |
Introduction
Doping nanotubes is an effective way to extensively modify their various properties [1-4]. The doped nanotubes can exhibit dramatic changes with respect to the undoped material [5]. For example BNNTs are mainly insulators. However, C-doped BNNTs have reduced electronic band gaps[6]. Carbon nanotubes doped with boron (B) and nitrogen (N) are also possible which can engineer the electronic band-gap of CNTs [7]. The doped nanotubes are also investigated for their potential applications as adsorbents[5, 8-10]. Methods based on PM6 and ONIOM methods allow to describe correctly the electronic structure and the reactivity of CNTs [11-14].
In study of scavenging characteristic of SWNTs for OH radicals, the semiempirical PM6 method was used for geometry optimization of carbon nanotubes [15].In this study, we have carried out systematic PM6 and ONIOM method to investigate the thermochemical aspects of aluminium doping of armchair and zigzag nanotubes . The doping reaction of aluminium with truncated (4,4) and (8,0) CNT, and BNNT evaluated thermochemically through PM6 and ONIOM studies.
Computational Details
We constructed the (8,0) zigzag and the (4,4) armchair CNT and BNNTs and optimized them using semiempirical quantum chemical method PM6 method and ONIOM method. Then we doped them with one atom of aluminium for more calculations All nanotube lengths were about 1-nm, open-ended and defect free. The two caps of all nanotubes were saturated with hydrogen atoms to simulate the effect of a longer nantube and to prevent the nanotube terminal bonds to dangle. Moreover, the hydrogen atoms decrease the cost of the calculation as well [16]. As Figure 1 reveals, for doping CNTs one carbon atom of nanotube was replaced by one aluminium atom. In BNNTs there are two atoms (B atom and N atom) which can be replaced with one Al atom.When boron atom was replaced with aluminum atom we named it as Al(B)-doped(4,4)BNNT, and for replacement of nitrogen atom with Al atom we named the resulted structure as Al(N)-doped(4,4)BNNT.When nitrogen atom of (4,4)BNNT replaced with Al atom in PM6 method there was a rough disarrangement in the structure of (4,4)BNNT. Due to this disarrangement we did not considered the Al(N)-doped(4,4)BNNT for Al-doping.When nitrogen atom of (8,0)BNNT replaced with Al atom in ONIOM method there was a rough disarrangement in the structure of (4,4)BNNT. Due to this disarrangement we did not considered the Al(N)-doped(4,4)BNNT for Al-doping.
The semiempirical methods, PM6 [17] applied to optimize the models, and also frequencies. These methods are implemented in the MOPAC program package[18].The ONIOM (Our N-layered Integrated molecular Orbital + molecular Mechanics) method developed by the author and his colleagues is an onion skin-like extrapolation method, allows to combine a variety of quantum mechanical methods as well as a molecular mechanics method in multiple layers[19-24]. Furthermore, frequency calculations were carried out to confirm their stability and to obtain thermochemical functions. The frequency calculations were performed at 1 atm of pressure. While we the temperature were set to increase by step of 5 degree of Kelvin starting from 298.15 K. To evaluate the doping reactions of NTs with aluminum atom we used the following reaction:
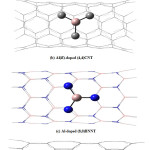 |
Figure 1: The optimized structures of (a) Al-doped (4,4)CNT (b) Al-doped (4,4)CNT (c) Al(B)-doped(8,0) BNNT (d) Al-doped (8,0)CNT
|
![]()
Results and Discussions
ONIOM Method
For BNNT and (4,4)CNT in all temperatures the doping reaction was endothermic and nonspontaneous. ∆rHdoping and ∆rGdoping trend for Al-doping reaction of (8,0)CNT is exothermic and nonspontaneous. There was an increasing trend in positive values for ∆rHdoping of (8,0)CNT.
Table 1: Calculated changes of thermodynamic functions of ( enthalpy kJ/mol, Gibbs free energy kJ/mol) of doping reactions at ONIOM and PM6 semiempirical level of theory.
| Temperature/K |
ONIOM |
PM6 |
||
|
Al(B)-Dpoed 44BNNT KJ/mol |
||||
| Delta(H) | Delta(G) | Delta(H) | Delta(G) | |
| 298.15 | 301.42 | 298.57 | 749.09 | 749.82 |
| 303.15 | 301.49 | 298.52 | 749.14 | 749.84 |
| 308.15 | 301.56 | 298.47 | 749.19 | 749.85 |
| 313.15 | 301.63 | 298.42 | 749.24 | 749.86 |
| 318.15 | 301.7 | 298.37 | 749.29 | 749.87 |
| 323.15 | 301.76 | 298.31 | 749.33 | 749.88 |
| 328.15 | 301.83 | 298.26 | 749.38 | 749.89 |
| 333.15 | 301.9 | 298.21 | 749.43 | 749.89 |
| 338.15 | 301.96 | 298.15 | 749.48 | 749.9 |
| 343.15 | 302.03 | 298.09 | 749.53 | 749.91 |
| 348.15 | 302.1 | 298.03 | 749.57 | 749.91 |
PM6 Method
we find that the general trend of changing enthalpies and Gibbs free energies follow the same trend. ∆rHdoping and ∆rGdoping for doped BNNTs and (4,4)CNT are positive (endothermic and nonspontaneous reactions) PM6 methods. At the PM6 method the ∆rHdoping and ∆rGdoping for Al-doping reaction of (8,0)CNT is exothermic and spontaneous.
Table 2: Calculated changes of thermodynamic functions of (enthalpy kJ/mol, Gibbs free energy kJ/mol) of doping reactions at ONIOM and PM6 semiempirical level of theory.
| Temperature/K |
ONIOM |
PM6 |
||
|
Al-dpoed 44CCNT KJ/mol |
||||
|
Delta(H) |
Delta(G) | Delta(H) |
Delta(G) |
|
| 298.15 | 333.29 | 323.89 | 442.15 | 433.74 |
| 303.15 | 333.48 | 323.73 | 442.33 | 433.61 |
| 308.15 | 333.67 | 323.57 | 442.51 | 433.47 |
| 313.15 | 333.85 | 323.4 | 442.68 | 433.32 |
| 318.15 | 334.05 | 323.23 | 442.86 | 433.17 |
| 323.15 | 334.24 | 323.06 | 443.03 | 433.01 |
| 328.15 | 334.43 | 322.89 | 443.2 | 432.85 |
| 333.15 | 334.62 | 322.71 | 443.38 | 432.69 |
| 338.15 | 334.81 | 322.53 | 443.56 | 432.53 |
| 343.15 | 335 | 322.35 | 443.75 | 432.36 |
| 348.15 | 335.2 | 322.16 | 443.92 | 432.2 |
Table 3: Calculated changes of thermodynamic functions of (enthalpy kJ/mol, Gibbs free energy kJ/mol) of doping reactions at ONIOM and PM6 semiempirical level of theory.
|
Temperature |
ONIOM |
PM6 |
||
|
Al(B)-doped 80 BNNT KJ/mol |
||||
| Delta(H) | Delta(G) |
Delta(H) |
Delta(G) | |
| 298.15 | 312.99 | 310.32 | 761.9 | 762.95 |
| 303.15 | 313.06 | 310.28 | 761.94 | 762.97 |
| 308.15 | 313.12 | 310.24 | 761.99 | 762.98 |
| 313.15 | 313.19 | 310.19 | 762.03 | 763 |
| 318.15 | 313.25 | 310.14 | 762.08 | 763.01 |
| 323.15 | 313.32 | 310.09 | 762.12 | 763.03 |
| 328.15 | 313.38 | 310.04 | 762.16 | 763.04 |
| 333.15 | 313.44 | 309.99 | 762.21 | 763.05 |
| 338.15 | 313.5 | 309.94 | 762.25 | 763.06 |
| 343.15 | 313.57 | 309.88 | 762.3 | 763.08 |
| 348.15 | 313.63 | 309.83 | 762.34 | 763.09 |
Table 4: Calculated changes of thermodynamic functions of ( enthalpy kJ/mol, Gibbs free energy kJ/mol) of doping reactions at ONIOM and PM6 semiempirical level of theory.
| Temperature/K |
ONIOM |
PM6 |
||
|
Al(B)-doped 80CNT KJ/mol |
||||
| Delta(H) | Delta(G) | Delta(H) | Delta(G) | |
| 298.15 | -0.01 | -7.11 | -169.66 | -183.68 |
| 303.15 | 0.18 | -7.24 | -169.41 | -183.91 |
| 308.15 | 0.37 | -7.38 | -169.16 | -184.16 |
| 313.15 | 0.56 | -7.49 | -168.92 | -184.4 |
| 318.15 | 0.75 | -7.61 | -168.67 | -184.65 |
| 323.15 | 0.93 | -7.78 | -168.42 | -184.9 |
| 328.15 | 1.12 | -7.89 | -168.17 | -185.16 |
| 333.15 | 1.31 | -8.03 | -167.91 | -185.42 |
| 338.15 | 1.5 | -8.17 | -167.66 | -185.69 |
| 343.15 | 1.69 | -8.31 | -167.41 | -185.95 |
| 348.15 | 1.88 | -8.46 | -167.15 | -186.23 |
Conclusions
We theoretically investigated the finitelength armchair and zigzag boron nitride,and carbon nanotubes for doping with Al atom. All nanotubes were single-walled and finite length with hydrogen saturation in the terminal atoms. The ONIOM and semiempirical quantum chemistry technique PM6 were used for the study.The results of ONIOM and PM6 showed the same trends for enthalpy and Gibbs free energy changes for Al-doping reactions of BNNTs and CNTs. The thermodynamic calculations based on the ONIOM and PM6 levels results showed that the zigzag (8,0)CNT is the best candidate for doping with aluminum atom at different studied temperature. The PM6 level of theory showed when nitrogen atom of (4,4)BNNT replaced with Al atom there was a rough disarrangement in the structure of (4,4)BNNT.The ONIOM method showed when nitrogen atom of (4,4)BNNT and (8,0)BNNT replaced with Al atom there was a rough disarrangement in the structure of (4,4)BNNT.
References
- Campos-Delgado, J., Maciel, I.O., Cullen, D.A., Smith, D.J., Jorio, A., Pimenta, M.A., et al. Chemical vapor deposition synthesis of N-, P-, and Si-doped single-walled carbon nanotubes. Acs Nano. 2010, 4, 1696-702.
CrossRef - Han, W., Bando, Y., Kurashima, K., Sato, T. Boron-doped carbon nanotubes prepared through a substitution reaction. Chemical physics letters. 1999, 299, 368-73.
CrossRef - Redlich, P., Loeffler, J., Ajayan, P., Bill, J., Aldinger, F., Rühle, M. B C N nanotubes and boron doping of carbon nanotubes. Chemical physics letters. 1996, 260, 465-70.
CrossRef - Borowiak-Palen, E., Pichler, T., Fuentes, G., Graff, A., Kalenczuk, R., Knupfer, M., et al. Efficient production of B-substituted single-wall carbon nanotubes. Chemical physics letters. 2003, 378, 516-20.
CrossRef - Li, F., Xia, Y., Zhao, M., Liu, X., Huang, B., Ji, Y., et al. Theoretical study of hydrogen atom adsorbed on carbon-doped BN nanotubes. Physics Letters A. 2006, 357, 369-73.
CrossRef - Terrones, M., Romo-Herrera, J., Cruz-Silva, E., López-Urías, F., Munoz-Sandoval, E., Velázquez-Salazar, J., et al. Pure and doped boron nitride nanotubes. Materials Today. 2007, 10, 30-8.
CrossRef - Meyyappan, M. Carbon nanotubes: science and applications, CRC press, 2004.
CrossRef - Wu, X., Yang, J., Hou, J., Zhu, Q. Deformation-induced site selectivity for hydrogen adsorption on boron nitride nanotubes. Physical Review B. 2004, 69, 153411.
CrossRef - Darkrim, F.L., Malbrunot, P., Tartaglia, G. Review of hydrogen storage by adsorption in carbon nanotubes. International Journal of Hydrogen Energy. 2002, 27, 193-202.
CrossRef - Dubot, P., Cenedese, P. Modeling of molecular hydrogen and lithium adsorption on single-wall carbon nanotubes. Physical Review B. 2001, 63, 241402.
CrossRef - Ormsby, J.L., King, B.T. The regioselectivity of addition to carbon nanotube segments. The Journal of organic chemistry. 2007, 72, 4035-8.
CrossRef - Erkoc, S., Özkaymak, S. Energetics of carbon nanotubes. The European Physical Journal D-Atomic, Molecular, Optical and Plasma Physics. 1998, 4, 331-3.
- Rochefort, A., Salahub, D.R., Avouris, P. Effects of finite length on the electronic structure of carbon nanotubes. The Journal of Physical Chemistry B. 1999, 103, 641-6.
CrossRef - Türker, L., Erkoç, Ş. Electronic properties of open-ended single wall carbon nanotubes. Journal of Molecular Structure: THEOCHEM. 2002, 577, 131-5.
CrossRef - Shukla, P., Mishra, P. Effects of diameter, length, chirality and defects on the scavenging action of single-walled carbon nanotubes for OH radicals: A quantum computational study. Chemical Physics. 2010, 369, 101-7.
CrossRef - Majidi, R., Ghafoori Tabrizi, K., Jalili, S. Effect of doping on electronic properties of double-walled carbon and boron nitride hetero-nanotubes. Physica B: Condensed Matter. 2009, 404, 3417-20.
CrossRef - Stewart, J.J. Optimization of parameters for semiempirical methods V: modification of NDDO approximations and application to 70 elements. Journal of Molecular modeling. 2007, 13, 1173-213.
CrossRef - JJP, S. MOPAC2012, Stewart computational chemistry. Colorado Springs, CO.
- Humbel, S.; Sieber, S.; Morokuma, K. J. Chem. Phys. 1996, 16, 1959.
CrossRef - Svensson, M.; Humbel, S.; Froese, R. D. J.; Matsubara, T.; Sieber, S.; Morokuma, K. J. Phys. Chem. 1996, 100, 19357.
CrossRef - Frisch, M. J. et al. Gaussian 98, Revision A.1; Gaussian, Inc.:Pittsburgh, PA, 1998; Frisch, M. J. et al. Gaussian 03, RevisionA.1; Pittsburgh, PA, 2003.
- Maseras, F. Top. Organomet. Chem. 1999, 4, 165.
CrossRef - Froese, R. D. J.; Morokuma, K. In The Encyclopedia of Computational Chemistry; Schleyer, P. v. R.; Allinger, N. L.; Clark, T.; Gasteiger, J.; Kollman, P. A.; Schaefer III, H. F.; Schreiner P. R., Eds.; John Wiley: Chichester, 1998; p 1245.
- Vreven, T.; Morokuma, K. J. Comp. Chem. 2000, 21, 1419.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.









